
The two-year anniversary point in any startup provides an opportunity to reflect on the surprises and unexpected turns in the road, many of which could not have been predicted. Solenic Medical’s second year was unlike any other experience I’ve had in my 20 years as an entrepreneur. If we had known what would happen in the spring of 2020, we might have made different decisions. Regardless, I am pleased to say that we made significant progress despite twelve months of COVID-driven lifestyle and work settings changes.
 I certainly did not expect to still be working primarily in virtual team mode. Solenic Medical launched in a remote work mode so it wasn’t unfamiliar, but I expected that by now, we would be working in an office somewhere, or at least sharing a few desks at a co-working office. Like many others, we have spent an enormous amount of time in Zoom meetings. I didn’t know this time last year how many meeting platforms we would need to accommodate including Zoom, Microsoft Teams, Google Meet, GoToMeeting, Webex, and others. I didn’t anticipate the need to transform my home office into a comfortable but professional meeting setting that looks good on video. In the past, no one cared where you set up to call into a virtual meeting, but a formal online conference requires a different environment. Fortunately, these days most participants welcome the occasional cat dropping into the meeting. On the positive side, we haven’t had to spend time with meeting location logistics and travel arrangements. In this new reality, we focus on the meeting approach and choosing the right virtual meeting platform, which sometimes can involve almost as much prep time as traveling. We travel far fewer miles now, but we burn up more bandwidth and we deal with the sticky reality of needing to upgrade internet service.
I certainly did not expect to still be working primarily in virtual team mode. Solenic Medical launched in a remote work mode so it wasn’t unfamiliar, but I expected that by now, we would be working in an office somewhere, or at least sharing a few desks at a co-working office. Like many others, we have spent an enormous amount of time in Zoom meetings. I didn’t know this time last year how many meeting platforms we would need to accommodate including Zoom, Microsoft Teams, Google Meet, GoToMeeting, Webex, and others. I didn’t anticipate the need to transform my home office into a comfortable but professional meeting setting that looks good on video. In the past, no one cared where you set up to call into a virtual meeting, but a formal online conference requires a different environment. Fortunately, these days most participants welcome the occasional cat dropping into the meeting. On the positive side, we haven’t had to spend time with meeting location logistics and travel arrangements. In this new reality, we focus on the meeting approach and choosing the right virtual meeting platform, which sometimes can involve almost as much prep time as traveling. We travel far fewer miles now, but we burn up more bandwidth and we deal with the sticky reality of needing to upgrade internet service.
Otherwise, at Solenic Medical, we have certainly experienced pandemic-related delays. Our first large animal study was scheduled for mid-March of last year at the University of Utah, was delayed until August 2020. COVID-19 did not create many more obstacles for the large animal surgery setting, but it did complicate our ability to get travel approval and ship our device to the lab. The operating environment remained business-as-usual, with professionals who were accustomed to mask requirements and sterilization procedures. COVID-19 also forced us to work smarter and plan even more carefully to maximize our efforts in local labs at UT Southwestern, as socially distancing in lab settings had an impact there, as well. Despite these challenges, we have managed to keep largely on track by spending more time preparing for the in-person activities and navigating around the new limitations on work hours and lab staffing restrictions.
Unlike changes we have seen in some infectious diseases due to COVID-19 issues, such as the drop in flu cases in the past year, we do not believe that the problem we are targeting has been affected. We witnessed a temporary decrease in elective surgeries including joint procedures, and potentially some delay by patients seeking help for infection problems, but no real direct impact from the pandemic itself.
Most prosthetic joint infections result from bacteria present in the body, bacteria introduced during the surgery itself, or bacteria introduced through subsequent procedures. In many cases, the bacteria exists elsewhere in the body. It waits for an opportunity, such as a weakened immune system, to attack a vulnerable spot, such as a surgical wound or prosthesis. Implant infections are notoriously difficult to treat. They’re caused by biofilm—densely packed colonies of bacteria embedded within a slimy extracellular matrix that resists antibiotics and the body’s immune system itself.
Solenic Medical's mission is to provide improved outcomes and quality of life for patients who undergo these implant procedures. With this goal in mind, we developed an innovative, non-invasive treatment for infected metallic implants in the body that takes advantage of the unique properties of alternating magnetic fields (AMF), using technology invented at the University of Texas Southwestern in Dallas. This breakthrough allows physicians to treat infections of implants from outside the body, reducing the need for costly and often risky surgical procedures, known as revision surgery. In revision surgeries, surgeons remove and replace implants usually in multiple surgeries. Unfortunately, revision procedures carry a high risk of failure of the implant, and a threat to the patient’s life.
Instead, we propose utilizing a non-invasive method to eradicate biofilm through our AMF device, as shown in the image below, where the heated implant surface destroys bacteria. Studies have shown six orders of magnitude reduction in biofilm when subjected to temperatures achievable by our technology, yet within range of temperatures seen in other procedures. For example our method operates at a temperature below the curing temperature of the typical cements used in implant procedures. This helps ensure the retention of existing implants and patients' mobility with much lower risk than the current standard of care.
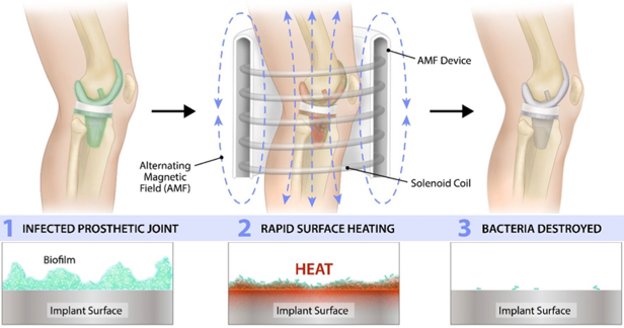 The most exciting part of the past two years has been talking to doctors and patients involved in the surgical world of prosthetic joints. Surgeons have called our device a “game changer” and “a home run for patients.” Patients have shown an eagerness to learn about a non-invasive alternative to additional surgeries. Our work has been informed by difficult conversations with individuals who described prosthetic joint infections, multiple surgeries to fight infections or even limb amputations due to a chronic joint infection. Our team is motivated by these conversations to bring this technology to market. Additionally, our 2020 designation by the FDA as a Breakthrough Device has the potential to speed our path to market. CMS's commitment to assign a reimbursement code during the first four years on the market will help ensure patients get access to this life-changing technology.
The most exciting part of the past two years has been talking to doctors and patients involved in the surgical world of prosthetic joints. Surgeons have called our device a “game changer” and “a home run for patients.” Patients have shown an eagerness to learn about a non-invasive alternative to additional surgeries. Our work has been informed by difficult conversations with individuals who described prosthetic joint infections, multiple surgeries to fight infections or even limb amputations due to a chronic joint infection. Our team is motivated by these conversations to bring this technology to market. Additionally, our 2020 designation by the FDA as a Breakthrough Device has the potential to speed our path to market. CMS's commitment to assign a reimbursement code during the first four years on the market will help ensure patients get access to this life-changing technology.
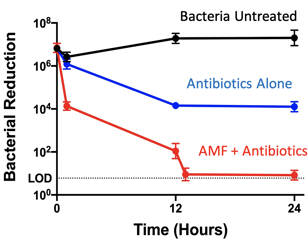 Though we are now approaching another wave of large animal trials, most of the past year has been spent testing the technology in test tubes with infections on metal coupons or rings, in gel models, through computer simulations, and through small animal trials. Despite some COVID-19-related delays, we’ve made consistent and positive progress in the labs with a wide range of tests and test conditions. One of the most conclusive graphs from these tests is shown below. The results of repeat testing show significant reduction in bacteria in conjunction with common antibiotics. The synergy between the use of AMF and antibiotics was significant across all tests. All in all, the results have been quite positive at this stage of testing. Those positive results prompted us to move forward our testing, conducting studies that verify safety and efficacy in preparation for the first in-human trials anticipated in Q3-2022.
Though we are now approaching another wave of large animal trials, most of the past year has been spent testing the technology in test tubes with infections on metal coupons or rings, in gel models, through computer simulations, and through small animal trials. Despite some COVID-19-related delays, we’ve made consistent and positive progress in the labs with a wide range of tests and test conditions. One of the most conclusive graphs from these tests is shown below. The results of repeat testing show significant reduction in bacteria in conjunction with common antibiotics. The synergy between the use of AMF and antibiotics was significant across all tests. All in all, the results have been quite positive at this stage of testing. Those positive results prompted us to move forward our testing, conducting studies that verify safety and efficacy in preparation for the first in-human trials anticipated in Q3-2022.
We achieved significant progress during a global pandemic, with the added complication of an atypical Texas freeze event in February that shut down power and disrupted water for millions. I figure that if we can accomplish this kind of progress during such a challenging time, we can certainly overcome any hurdles ahead.
Among those hurdles will be the need to continually invest in our intellectual property, with three filed patents and more to come, and the need to expand our professional team. We are excited about these two areas because we know we are building both on a strong base.
We certainly expect more surprises, and we wouldn’t be doing our job if we weren’t ready for them. But our first two years have prepared us for what lies ahead. Upcoming challenges include a wider variety of safety and efficacy tests, moving from 5 large animals in tests to 25-30, device design freeze and build. Then the big, our fundraising round to pay for those steps by raising a couple million dollars. No problem, we’ve got this with or without masks and social distancing requirements!
In the next two years, we expect to reach the core of our mission, which is to be in medical facilities improving outcomes and quality of life for patients undergoing metallic implant procedures. Achieving this goal will be an outstanding achievement in the young life of Solenic Medical.

